Abstract
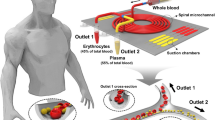
To demonstrate a highly efficient microfluidic system for motile sperm sorting, we designed a novel microfluidic device with three inlets and three outlets. Utilizing the different flow rate as a basic background, the characteristics of laminar flow that has three parallel flows within a microfluidic chip. In the main channel, only motile sperm can break through boundary layer from its original laminar flow, because motile sperm has a property of swim freely. Therefore, this mechanism can separate motile sperm from no motile sperm and other particles. Motile sperm from the upper and lower inlets in our device can break through the stream boundary to the collection reservoir. Through microscopic observation, fluorescent images of sperm with varied motility indicated that the laminar streams are similar to results from CFDRC simulation. To confirm the real sorting situation, we identified and quantified the proportions of living and dead sperm after sorting through flow cytometric analysis. We compared the reservoir sorting area and waste area to realize that efficiency is improved. The advantages of our device are including stable field, sample double processing and main channel designed gradually wider which is beneficial with increase sorting possibility. The result showed that sperm collected via our sorting device have greater activity as sperm motility.







Similar content being viewed by others
References
Aitken RJ, Clarkson JS (1988) Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl 9(6):367–376
Bonduelle M, Camus M, De Vos A, Staessen C, Tournaye H, Van Assche E, Verheyen G, Devroey P, Liebaers I, Van Steirteghem A (1999) Seven years of intracytoplasmic sperm injection and follow-up of 1987 subsequent children. Hum Reprod 14:243–264
Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C (2007) Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev 4:CD004507
Brown M, Wittwer C (2000) Flow cytometry: principles and clinical applications in hematology. Clin Chem 46(8):1221–1229
Chen C-H, Chuang S-C, Su H-C, Hsu W-L, Yew T-R, Chang Y-C, Yeh S-R, Yao D-J (2011) A three-dimensional flexible microprobe array for neural recording assembled through electrostatic actuation. Lab Chip 11(9):1647–1655
Cho BS, Schuster TG, Zhu XY, Chang D, Smith GD, Takayama S (2003) Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem 75(7):1671–1675
Donnelly ET, O’Connell M, McClure N, Lewis SEM (2000) Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum Reprod 15(7):1552–1561
Englert Y, Vandenbergh M, Rodesch C, Bertrand E, Biramane J, Legreve A (1992) Comparative auto-controlled study between swim-up and percoll preparation of fresh semen samples for invitro fertilization. Hum Reprod 7(3):399–402
Henkel RR, Schill W-B (2003) Sperm preparation for ART. Reprod Biol Endocrinol: RB&E 1(108–108):2003
Horsman KM, Barker SLR, Ferrance JP, Forrest KA, Koen KA, Landers JP (2005) Separation of sperm and epithelial cells in a microfabricated device: Potential application to forensic analysis of sexual assault evidence. Anal Chem 77(3):742–749
Huang WH, Cheng W, Zhang Z, Pang DW, Wang ZL, Cheng JK, Cui DF (2004) Transport, location, and quantal release monitoring of single cells on a microfluidic device. Anal Chem 76(2):483–488
Huang H, Fu H, Tsing H, Huang H, Li C, Yao D (2014a) Motile human sperm sorting by an integrated microfluidic system. J Nanomed Nanotechnol 5(199):2
Huang H-Y, Wu T-L, Huang H-R, Li C-J, Fu H-T, Soong Y-K, Lee M-Y, Yao D-J (2014b) Isolation of motile spermatozoa with a microfluidic chip having a surface-modified microchannel. Jala 19(1):91–99
Kricka LJ (1998) Miniaturization of analytical systems. Clin Chem 44(9):2008–2014
Kricka LJ, Faro I, Heyner S, Garside WT, Fitzpatrick G, McKinnon G, Ho J, Wilding P (1997) Micromachined analytical devices: microchips for semen testing. J Pharm Biomed Anal 15(9–10):1443–1447
Lin TH, Yao DJ (2012) Applications of EWOD systems for DNA reaction and analysis. J Adhes Sci Technol 26(12–17):1789–1804
Lin H-C, Liu Y-J, Yao D-J (2010) Core-shell droplets for parallel dna ligation of an ultra-micro volume using an EWOD microfluidic system. Jala 15(3):210–215
Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P (2002) Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod 17(5):1257–1265
McCormack MC, McCallum S, Behr B (2006) A novel microfluidic device for male subfertility screening. J Urol 175(6):2223–2227
Mortimer D (1991) Sperm preparation techniques and iatrogenic failures of invitro fertilization. Hum Reprod 6(2):173–176
Slentz BE, Penner NA, Regnier FE (2002) Capillary electrochromatography of peptides on microfabricated poly (dimethylsiloxane) chips modified by cerium (IV)-catalyzed polymerization. J Chromatogr A 948(1):225–233
Steptoe PC, Edwards RG (1978) Birth after the reimplantation of a human embryo. Lancet 312(8085):366
Verpoorte E (2002) Microfluidic chips for clinical and forensic analysis. Electrophoresis 23(5):677–712
Zini A, Finelli A, Phang D, Jarvi K (2000) Influence of semen processing technique on human sperm DNA integrity. Urology 56(6):1081–1084
Acknowledgments
The authors would like to acknowledge the financial support provided by NTHU/CGMH collaboration funds (CMRPG30082, CMRPG380121 and CMRPG390441), and part of fund comes from National Science Council of Taiwan (NSC 101-2221-E-007-099-MY3 and NSC 102-2120-M-002-009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, HY., Huang, PW. & Yao, DJ. Enhanced efficiency of sorting sperm motility utilizing a microfluidic chip. Microsyst Technol 23, 305–312 (2017). https://doi.org/10.1007/s00542-015-2495-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-015-2495-6