Novel Discotic Boroxines: Synthesis and Mesomorphic Properties
Abstract
: A new synthetic approach to highly substituted triphenylboroxines 11 is described. Their mesomorphic properties were investigated by differential scanning calorimetry (DSC), polarizing optical microscopy (POM) and X-ray diffraction (SAXS, WAXS). The tris(3,4,5-trialkyloxy)phenyl functionalized derivatives 11b–e showed broad mesophases for a minimum alkyl chain length of C9. The phase widths ranged from 110 K to 77 K near room temperature, thus decreasing with enhanced alkyl chain lengths. Textures observed under POM indicated a columnar hexagonal (Colh) mesophase symmetry that was confirmed by X-ray diffraction experiments.1. Introduction
The discovery of stable liquid crystalline phases formed by disk-shaped molecules with long alkyl chains in their periphery is generally dated to the seminal work of Chandrasekar published in 1977 [1]. Since then discotic liquid crystals have attracted the attention of many research groups worldwide [2–4]. Due to the one dimensional charge and ion transport in the columnar mesophase, the ability of liquid crystals (LCs) to self-heal structural defects by thermal annealing and the ease of processing via spin coating, drop casting and other solution processing methods highly useful applications [5–7] could berealized such as organic solar cells [8], organic field effect transistors [3] and organic light emitting diodes [9].
From a molecular perspective, several scaffolds have turned out to be successful candidates for the nanosegregation, which ultimately led into the formation of columnar mesophases. Besides tri- and hexasubstituted benzenes, in particular triphenylenes [10], perylenes [11], hexa-peri-hexabenzocoronenes [12,13], porphyrins [14,15], phthalocyanines [15], quinoxalines and other aza analogues of polycyclic aromatic hydrocarbons were used as mesogenic subunits. However, not only disk-shaped mesogens, but also non-conventional [16] and supramolecular and hydrogen bonded liquid crystals [17] can self-assemble into columnar aggregates. While a variety of LC materials containing heterocyclic 6-membered rings have been synthesized [18], e.g., triazines such as 1 [19–21] or cyclotriphosphazenes such as 2 [22,23] (Figure 1), surprisingly little is known about liquid crystalline boroxines, the cyclic trimers of organoboronic acids. Aryl boronic acids are valuable reagents for a number of metal-catalyzed reactions, the most prominent one thereof is certainly the Suzuki-Miyaura cross-coupling reaction [24–28]. Furthermore, boroxines may serve as useful building blocks for flame retardants, lithium ion battery materials and covalent organic frameworks [29,30]. The only example considering mesomorphism was a report by Preece and coworkers who studied a series of tris(alkoxyphenyl)boroxines 3, which, however, did not reveal any mesophases (Figure 1) [31].
Based on this precedence we anticipated that the attachment of additional alkoxy groups at the aryl rings should favor nanosegregation and thus induce mesophase formation. The results towards this goal are reported below.
2. Results and Discussion
2.1. Synthesis of Boroxines
First, we intended to attach alkyloxy groups with chains lengths of C12-C18 at the phenyl rings in boroxine 3 following known procedures [32–35]. However, only 3g (R1 = C12H25) could be isolated in 30% yield, whereas boroxines with alkyl chain lengths >12 were not obtained as a problem of poor solubility of the precursor boronic acid (for details see Supplementary Information). Derivative 3g did not show mesomorphic properties.
Following our envisioned strategy, 4-bromo-1,2-dialkoxybenzenes 4 [36,37] were lithiated with n-BuLi, THF at −78 °C, followed by treatment with B(OMe)3 and then directly hydrolyzed by addition of aqueous HCl to the mixture. No trace of the boronic acids was detected, but the boroxines 5 were isolated (Scheme 1). Their yields decreased considerably with increasing chain lengths from 51% for 5a to 7% for 5d due to purification problems. Column chromatography led to decomposition, thus leaving recrystallization as only method to purify 5. With increasing chain length the solubility of dialkoxybromides 4, boronic acids 6 and boroxines 5 was more and more alike. Therefore, the crude product of 5e with two C16H33 groups at the phenyl ring was found as an inseparable mixture of those three and no pure product could be obtained.
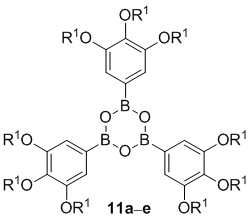
The synthesis of tris(3,4,5-trialkoxyphenyl)boroxines 11 commenced with the 5-bromo-1,3,4-trialkoxybenzenes 7 [38–41]. Their conversion into the boronic acids according to the method described in Scheme 1 turned out to be problematic, because inseparable mixtures of boronic acids and boroxines were obtained. This was also true, when the initial bromo-lithium exchange was replaced by formation of a Grignard species instead. Therefore, an alternative method was applied (Scheme 2).
Bromides 7 were treated with n-BuLi, followed by addition of isopropoxypinacolborolane [42] to yield the corresponding pinacolborolanes 8 [38] which were directly treated with bisethanolamine in i-PrOH. The resulting diethanolamine complexes 9 precipitated from the solution and after filtration, they were hydrolyzed without further purification with HCl in THF giving a mixture of boroxines 11 and boronic acids 10. Refluxing this mixture with pyridine in Et2O [43] followed by hydrolysis with HCl in Et2O provided the desired free boroxines 11 quantitatively. Noteworthy, this strategy did not need purification at any step. Only pinacolborolanes 8 were deprotected to complexes 9 which precipitated in analytically pure form (shown for 9e in the Supplementary Information). The subsequent two reaction steps proceeded in quantitative yield without any impurities. The overall yields of 11 starting from 7 were in the range of 20%–40%. The solid products 11 were air-stable, however, storage in solvents containing traces of water led to slow decomposition to the corresponding boronic acids 10.
2.2. Mesomorphic Properties of Boroxines
The liquid crystalline properties of compounds 5 and 11 were studied by differential scanning calorimetry (DSC), polarizing optical microscopy (POM) and X-ray diffraction (XRD: wide-angle X-ray scattering (WAXS), small-angle X-ray scattering (SAXS)).
All compounds 5a–d bearing three 3,4-dialkoxyphenyl substituents did not show any mesomorphism. In contrast, boroxines 11 with additional alkoxy group turned out to be mesogenic with exception of derivative 11a with C8 alkyloxy side chains. The results obtained from DSC measurements are summarized in Table 1 and Figure 2.
Boroxines 11b–e displayed broad mesophases near room temperature. For boroxine 11b with C9 alkyloxy side chains a liquid crystalline phase between 25 °C and 135 °C was observed. With increasing chain lengths melting points continuously rose up to 49 °C (11e) and clearing points receded to 123 °C resulting in reduced phase widths from 100 K (11c) to 77 K (11e).
Considering the DSC traces of 11b–11e identical behaviour of all derivatives during heating and cooling cycle is evident. As example, DSC curves of 11e are shown in Figure 3 (for further DSC curves see Supplementary Information). Melting points were clearly visible while clearing point peaks were less intensive and very broad. Hence, peak temperatures are given in Table 1. Upon cooling both melting and clearing point displayed a supercooling of phase transitions. Clearing points were slightly affected. Boroxine 11b shows the largest shift of 6 K from 135 °C upon heating to 129 °C upon cooling. The melting points of 11b and 11d, however, were significantly shifted by 18 K from 25 °C (11b) and 42 °C (11d) to 7 °C and 24 °C, respectively.
Under the microscope boroxines 11b–11e behaved similarly. Upon cooling from the isotropic liquid all derivatives formed homeotropic areas with few defects (Figure 4 top).
Presumably, the boron atoms coordinate at the oxygen atoms of the glass surface, resulting in aligned mesophases perpendicular to the surface. Therefore, the glass slides were treated with trimethylchlorosilane prior to use. In this way, broken fan-shaped textures being typical for columnar mesophases could be observed for boroxines 11b–11e under the POM (Figure 4 bottom).
In order to get further insight into the phase geometry, derivatives 11b–e were investigated by XRD (SAXS and WAXS). Figure 5 shows both the SAXS profile and WAXS pattern exemplarily for boroxine 11e. In the small-angle region three reflections are visible in a ratio of 1:1/ :1/2 which were indexed as (10), (11) and (20) [44]. This diffraction pattern is typical for columnar hexagonal (Colh) phase geometries. In the wide-angle region a diffuse halo was observed, which is generated through the interaction of the molten-like alkyl chains. In the case of derivatives 11b–d, however, only the (10) reflection and the diffuse halo were clearly visible in the diffractograms. Due to identical molecule geometry also Colh mesophases are assumed for 11b–d (for XRD data see Supplementary Information).
3. Experimental Section
3.1. Materials
All reagents were used as purchased from the suppliers without further purification. Solvents were dried and distilled under nitrogen prior to use and unless otherwise stated all reactions were carried out under nitrogen atmosphere with Schlenk-type glassware.
3.2. General Procedure for the Synthesis of Tris(3,4,5-Trialkoxyphenyl)boroxines (11)
To a solution of the appropriate 7 (4.225 mmol) in abs. THF (150 mL) at −78 °C was added n-BuLi (4.50 mL, 7.2 mmol, 1.6 M in n-hexane, Merck KGaA, Darmstadt, Germany) and the reaction mixture was stirred for 1 h. Then isopropyl pinacol borate (1.32 mL, 1.20 g, 6.34 mmol, Sigma-Aldrich, Steinheim, Germany) was added and the reaction mixture stirred for a further 1 h at −78 °C. After warming to room temperature over 24 h, the reaction was terminated by addition of NH4Cl (50 mL, saturated solution) and stirring for 1 h. The resulting aqueous suspension was extracted with Et2O (3 × 50 mL). The combined organic layers were washed with H2O (2 × 100 mL) and brine (80 mL) and dried (MgSO4). The solvent was removed under vacuum and the crude product 8 was directly used for the next step.
The crude appropriate pinacolborolane 8 was dissolved in a minimal amount of isopropanol, diethanolamine (0.85 mL, 0.89 g, 8.45 mmol, calcd for quantitative yield in the previous step, Sigma-Aldrich, Steinheim, Germany) was added, and the mixture was stirred for 24 h at ambient temperature. The suspension was filtered giving the boronic acid diethanolamine complexes 9 as white solids. Those were dissolved in THF (15 mL) and stirred with HCl (7 mL, 2 M) for 2 h at room temperature. The resulting aqueous suspension was extracted with Et2O (3 × 30 mL). The combined organic layers were washed with H2O (2 × 500 mL) and brine (50 mL) and dried (MgSO4). The solvent was removed under vacuum giving mixtures of boronic acids 10 and the appropriate boroxines 11 in good overall yield.
The respective mixture of 10 and 11 (0.3 mmol, calcd for the maximum amount of 10) was dissolved in abs. Et2O and, together with mol sieves (4 Å) to trap resulting water, heated to reflux. Pyridine (abs., 0.1 mL, 0.095 g, 1.2 mmol) was added and the mixture was stirred for additional 20 min. The solution was then cooled with ice (0 °C) and HCl·Et2O (pH = 1) was added. After 10 min, the precipitate was collected on a glass fritted funnel and the filtrate was fully evaporated in vacuo to give the desired boroxines 11 as colorless solids in quantitative yield.
3.2.1. Tris(3,4,5-Trioctyloxyphenyl)boroxine (11a)
1H NMR (500 MHz, CDCl3): δ = 0.83–0.95 (m, 27H, CH3), 1.23–1.43 (m, 72H, CH2), 1.44–1.56 (m, 18H, OCH2CH2CH2), 1.72–1.90 (m, 18H, OCH2CH2), 4.01–4.14 (m, 18H, OCH2), 7.39 (s, 6H, 2-H) ppm. 13C NMR (126 MHz, CDCl3): δ = 14.11, 14.12 (CH3), 22.70, 22.72, 26.1, 26.2, 29.3, 29.40, 29.46, 29.55, 29.56, 30.39, 31.87, 31.93 (CH2), 69.2, 73.5 (OCH2), 113.9 (C-2), 142.7 (C-4), 152.9 (C-3) ppm. FT-IR (ATR): = 2927 (w), 2855 (w), 1574 (w), 1467 (w), 1417 (w), 1333 (s, B-O), 1110 (w), 904 (s), 726 (vs, BX), 649 (s) cm−1 (BX denotes the anhydride band [45]). MS (MALDI-TOF): m/z calcd. for [C90H159B3O12]+ 1465.21, found 1465.04. Anal. calcd. for C90H159B3O12 (1465.68 g·mol−1): C 73.75, H 10.93; found: C 73.39, H 11.02.
3.2.2. Tris(3,4,5-Trinonyloxyphenyl)boroxine (11b)
1H NMR (500 MHz, CDCl3): δ = 0.85–0.91 (m, 27H, CH3), 1.21–1.43 (m, 90H, CH2), 1.45–1.56 (m, 18H, OCH2CH2CH2), 1.73–1.90 (m, 18H, OCH2CH2), 4.04–4.13 (m, 18H, OCH2), 7.39 (s, 6H, 2-H) ppm. 13C NMR (126 MHz, CDCl3): δ = 14.12 14.13 (CH3), 22.71, 22.72, 26.1, 26.2, 29.3, 29.4, 29.52, 29.55, 29.62, 29.66, 29.7, 30.4, 31.94, 31.97 (CH2), 69.2, 73.5 (OCH2), 113.9 (C-2), 142.7 (C-4), 152.9 (C-3) ppm. FT-IR (ATR): = 2958 (w), 2924 (w), 2853 (w), 1575 (w), 1467 (w), 1417 (w), 1334 (s, B-O), 1261 (w), 1213 (w), 1111 (w), 1021 (w), 905 (s), 849 (w), 804 (w), 725 (vs, BX), 649 (w) cm−1. MS (MALDI-TOF): m/z calcd. for [C99H177B3O12]+ 1591.35, found 1591.79. Anal. calcd. for C99H177B3O12 (1591.92 g·mol−1): C 74.70, H 11.21; found: C 74.30, H 11.30.
3.2.3. Tris(3,4,5-Tridecyloxyphenyl)boroxine (11c)
1H NMR (500 MHz, CDCl3): δ = 0.82–0.96 (m, 27H, CH3), 1.17–1.45 (m, 108H, CH2), 1.45–1.63 (m, 18H, OCH2CH2CH2), 1.71–1.93 (m, 18H, OCH2CH2), 4.02–4.13 (m, 18H, OCH2), 7.39 (s, 6H, 2-H) ppm. 13C NMR (126 MHz, CDCl3): δ = 14.1 (CH3), 22.7, 26.1, 26.2, 29.40, 29.41, 29.51, 29.54, 29.60, 29.65, 29.7, 29.8, 30.38, 31.93, 31.95 (CH2), 69.22, 73.50 (OCH2), 113.9 (C-2), 142.7 (C-4), 152.9 (C-3) ppm. FT-IR (ATR): = 2958 (w), 2923 (w), 2853 (w), 1574 (w), 1466 (w), 1417 (w), 1333 (s, B-O), 1261 (w), 1212 (w), 1112 (w), 1014 (w), 905 (s), 801 (w), 723 (vs, BX), 645 (w) cm−1. MS (MALDI-TOF): m/z calcd. for [C108H195B3O12]+ 1717.49, found 1717.89. Anal. calcd. for C108H195B3O12 (1718.16 g·mol−1): C 75.50, H 11.44; found: C 75.25, H 11.46.
3.2.4. Tris(3,4,5-Triundecyloxyphenyl)boroxine (11d)
1H NMR (500 MHz, CDCl3): δ = 0.80–0.94 (m, 27H, CH3), 1.20–1.43 (m, 126H, CH2), 1.45–1.58 (m, 18H, OCH2CH2CH2), 1.70–1.93 (m, 18H, OCH2CH2), 4.02–4.13 (m, 18H, OCH2), 7.39 (s, 6H, 2-C) ppm. 13C NMR (126 MHz, CDCl3): δ = 14.1 (CH3), 22.7, 26.1, 26.2, 29.4, 29.50, 29.54, 29.6, 29.71, 29.75, 29.76, 30.4, 31.94, 31.96 (CH2), 69.2, 73.50 (OCH2), 113.9 (C-2), 142.7 (C-4), 152.9 (C-5) ppm. FT-IR (ATR): = 2958 (w), 2920 (s), 2851 (w), 1575 (w), 1506 (w), 1467 (w), 1419 (s), 1336 (vs, B-O), 1261 (w), 1241 (w), 1212 (w), 1172 (w), 1111 (s), 1021 (w), 845 (w), 802 (w), 721 (s, BX), 667 (w), 648 (w) cm−1. MS (MALDI-TOF): m/z calcd. for [C117H213B3O12]+ 1843.63, found 1843.49. Anal. calcd. for C117H213B3O12 (1844.41 g·mol−1): C 76.19, H 11.64; found: C 76.39, H 11.33.
3.2.5. Tris(3,4,5-Tridodecyloxyphenyl)boroxine (11e)
1H NMR (500 MHz, CDCl3): δ = 0.88 (m, 27H, CH3), 1.17–1.44 (m, 144H, CH2), 1.45–1.61 (m, 18H, OCH2CH2CH2), 1.74–1.90 (m, 18H, OCH2CH2), 4.00–4.16 (m, 18H, OCH2), 7.39 (s, 6H, 2-H) ppm. 13C NMR (126 MHz, CDCl3): δ = 14.12 (CH3), 22.7, 26.1, 26.2, 29.4, 29.53, 29.55, 29.6, 29.72, 29.77, 29.78, 30.4, 31.9 (CH2), 69.23, 73.50 (OCH2), 113.9 (C-2), 142.7 (C-4), 152.9 (C-3) ppm. FT-IR (ATR): = 2957 (w), 2919 (s), 2850 (s), 1575 (w), 1467 (w), 1419 (s), 1336 (vs, B-O), 1260 (w), 1213 (w), 1113 (s), 1012 (w), 907 (w), 846 (w), 799 (w), 736 (s), 720 (s, BX), 648 (w), 578 (w) cm−1. MS (MALDI-TOF): m/z calcd. for [C126H231B3O12-H]+ 1969.77, found 1968.80. Anal. calcd. for C126H231B3O12 (1970.65 g·mol−1): C 76.80, H 11.82; found: C 77.02, H 11.92.
3.3. Instrumental Analysis
The following instruments were used for characterization of the compounds. NMR: Bruker Avance 500 (1H, 500 MHz; 13C, 126 MHz) and Bruker Avance 300 (1H, 300 MHz; 13C, 75 MHz). 1H and 13C NMR spectra were referenced to tetramethylsilane (TMS, Sigma-Aldrich, Steinheim, Germany) (δH = 0.0 ppm, δC = 0.0 ppm) as an internal standard. Unless otherwise stated, spectra were recorded at room temperature. Assignment of the resonances was supported by chemical shift calculations and 2D experiments (COSY and HMBC). Elemental analyses: Carlo Erba Strumentazione Elemental Analyzer, Modell 1106. IR: Bruker Vector 22 FT-IR Spectrometer with MKII golden gate single reflection Diamant ATR system. MS (ESI): Bruker Daltonics microTOF-Q spectrometer. Polarizing optical microscopy: Olympus BX50 polarizing microscope combined with a Linkam TP93 central controller. X-ray diffraction (WAXS, SAXS regions): Bruker AXS Nanostar C diffractometer employing Ni-filtered CuKα radiation (λ = 1.5418 Å), standard stationary temperature control unit for temperature programs. Differential scanning calorimetry (DSC): Mettler-Toledo DSC 822e. Flash chromatography was performed on silica gel, grain size 40–63 μm (Fluka) and aluminium sheets precoated with silica gel 60 F254 (Merck) were used for thin layer chromatography.
4. Conclusions
A new and easy approach towards discotic boroxines and study of their mesomorphic properties is reported. Pure boroxines 11 were accessible starting from 5-bromo-1,3,4-trialkoxybenzenes 7 via four-step reaction which did not need purification of intermediates.
Derivatives 5 bearing three dialkyloxyphenyl groups are found to be non-mesomorphic. In contrast, linkage of three trialkyloxyphenyl substituents gave boroxines 11 displaying liquid crystalline behaviour. A minimum of C9 alkyl chain length (11b) turned out to be necessary for mesophase formation because 11a with C8 alkyl chains is non-mesogenic. The phase widths ranged from 77 K (11e) to 110 K (11b) near room temperature, thus decreasing with enhanced alkyl chain lengths. Under POM, fan-shaped textures typical for columnar mesophases were observed for all boroxines 11b–e. The phase geometry was further supported by X-ray diffraction showing in the small-angle region the typical diffraction pattern of columnar hexagonal mesophases.
Acknowledgments
Generous financial support by the Ministerium für Wissenschaft, Forschung und Kunst des Landes Baden-Württemberg, the Bundesministerium für Bildung und Forschung (shared instrumentation grant No. 01 RI 05177), and the Fonds der Chemischen Industrie is gratefully acknowledged.
Author Contributions
Tobias Wöhrle performed the synthesis and characterization. Tobias Wöhrle, Angelika Baro and Sabine Laschat prepared the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandrasekhar, S.; Sadashiva, B.K.; Suresh, K.A. Liquid crystals of disc-like molecules. Pramana 1977, 9, 471–480. [Google Scholar]
- Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; et al. Discotic liquid crystals: from tailor-made synthesis to plastic electronics. Angew. Chem. Int. Ed 2007, 46, 4832–4887. [Google Scholar]
- Bushby, R.J.; Kawata, K. Liquid crystals that affected the world: Discotic liquid crystals. Liq. Cryst 2011, 38, 1415–1426. [Google Scholar]
- Kumar, S. Design concepts and synthesis of discotic liquid crystals. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T.K., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 4, pp. 467–520. [Google Scholar]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Discotic liquid crystals: A new generation of organic semiconductors. Chem. Soc. Rev 2007, 36, 1902–1929. [Google Scholar]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater 2007, 6, 929–938. [Google Scholar]
- Kaafarani, B.R. Discotic liquid crystals for opto-electronic applications. Chem. Mater 2011, 23, 378–396. [Google Scholar]
- Kumar, S. Discotic liquid crystals for solar cells. Curr. Sci 2002, 82, 256–257. [Google Scholar]
- Kopitzke, J.; Wendorff, J.H. Diskotische Flüssigkristalle: Materialien für die Optoelektronik. Chem. Unserer Zeit 2000, 34, 4–16. (In German). [Google Scholar]
- Kumar, S. Recent developments in the chemistry of triphenylene-based discotic liquid crystals. Liq. Cryst 2004, 31, 1037–1059. [Google Scholar]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun 2004, 1564–1579. [Google Scholar]
- Seyler, H.; Purushothaman, B.; Jones, D.J.; Holmes, A.B.; Wong, W.W.H. Hexa-peri-hexabenzocoronene in organic electronics. Pure Appl. Chem 2012, 84, 1047–1067. [Google Scholar]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as potential material for electronics. Chem. Rev 2007, 107, 718–747. [Google Scholar]
- Fox, M.A.; Bard, A.J.; Pan, H.-L.; Liu, C.-Y. Functionalized porphyrin discotic liquid crystals: Photoinduced charge separation and trapping. J. Chin. Chem. Soc 1993, 40, 321–327. [Google Scholar]
- Eichhorn, H. Mesomorphic phthalocyanines, tetraazaporphyrins, porphyrins and triphenylenes as charge-transporting materials. J. Porphyr. Phthalocyanines 2000, 4, 88–102. [Google Scholar]
- Tschierske, C. Liquid crystal engineering—New complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev 2007, 36, 1930–1970. [Google Scholar]
- Kato, T.; Yasuda, T.; Kamikawa, Y.; Yoshio, M. Self-assembly of functional columnar liquid crystals. Chem. Commun 2009, 729–739. [Google Scholar]
- Roy, B.; de, N.; Majumdar, K.C. Advances in metal-free heterocycle-based columnar liquid crystals. Chemistry 2012, 18, 14560–14588. [Google Scholar]
- Majumdar, K.C.; De, N.; Roy, B.; Bhaumik, A. Synthesis and mesophase characterisation of a series of new triazine-based disc-shaped molecules. Liq. Cryst 2010, 37, 1459–1464. [Google Scholar]
- Kohlmeier, A.; Vogel, L.; Janietz, D. Multiple hydrogen bonded mesomorphic complexes between complementary 1,3,5-triazine und pyrimidine derivatives. Soft Matter 2013, 9, 9476–9486. [Google Scholar]
- Castelar, S.; Barberá, J.; Marcos, M.; Romero, P.; Serrano, J.-L.; Golemme, A.; Termine, R. Supramolecular dendrimers based on the self-assembly of carbazole-derived dendrons and triazine rings: Liquid crystals, photophysical and electrochemical properties. J. Mater. Chem. C 2013, 1, 7321–7332. [Google Scholar]
- Barberá, J.; Bardaji, M.; Jiménez, J.; Laguna, A.; Martinez, M.P.; Oriol, L.; Serrano, J.L.; Zaragozano, I. Columnar mesomorphic organizations in cyclotriphosphazenes. J. Am. Chem. Soc 2005, 127, 8994–9002. [Google Scholar]
- Barberá, J.; Jiménez, J.; Laguna, A.; Oriol, L.; Pérez, S.; Serrano, J.L. Cyclotriphosphazene as a dendritic core for the preparation of columnar supermolecular liquid crystals. Chem. Mater 2006, 18, 5437–5445. [Google Scholar]
- Coeffard, V.; Moreau, X.; Thomassigny, C.; Greck, C. Übergangsmetallfreie Aminierung von Arylboronsäuren und deren Derivaten. Angew. Chem 2013, 125, 5794–5796. [Google Scholar]
- Suzuki, A. Cross-coupling reactions of organoboranes: an easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed 2011, 50, 6722–6737. [Google Scholar]
- Miyaura, N. Metal-catalyzed reactions of organoboronic acids and esters. Bull. Chem. Soc. Jpn 2008, 81, 1535–1553. [Google Scholar]
- Miyauro, N. Organoboron compounds. Topics Curr. Chem 2002, 219, 11–59. [Google Scholar]
- Hayashi, T. Rhodium-catalyzed asymmetric 1,4-addition of organoboronic acids and their derivatives to electron deficient olefins. Synlett 2001, 879–887. [Google Scholar]
- Tokunaga, Y. Boroxine chemistry: From fundamental studies to applications in supramolecular and synthetic organic chemistry. Heterocycles 2013, 87, 911–1021. [Google Scholar]
- Korich, A.L.; Iovine, P.M. Boroxine chemistry and applications: A perspective. Dalton Trans 2010, 39, 1423–1431. [Google Scholar]
- Belloni, M.; Manickam, M.; Wang, Z.-H.; Preece, J.A. Toward boronate ester mesogenic structures. Mol. Cryst. Liq. Cryst 2003, 399, 93–114. [Google Scholar]
- An, P.; Shi, Z.-F.; Dou, W.; Cao, X.-P.; Zhang, H.-L. Synthesis of 1,4-bis[2,2-bis (4-alkoxyphenyl) vinyl]benzenes and side chain modulation of their solid-state emission. Org. Lett 2010, 12, 4364–4367. [Google Scholar]
- Zou, Y.; Yi, T.; Xiao, S.; Li, F.; Li, C.; Gao, X.; Wu, J.; Yu, M.; Huang, C. Amphiphilic diarylethene as a photoswitchable probe for imaging living cells. J. Am. Chem. Soc 2008, 130, 15750–15751. [Google Scholar]
- Lindner, N.; Kölbel, M.; Sauer, C.; Diele, S.; Jokiranta, J.; Tschierske, C. Formation of columnar and cubic mesophases by calamitic molecules: novel amphotropic biphenyl derivatives. J. Phys. Chem. B 1998, 102, 5261–5273. [Google Scholar]
- Kwiatkowski, M.; Chattopadhyaya, J. The 9-(4-octadecyloxyphenylxanthen)-9-yl-group. A new acid-labile hydroxyl protective group and its application in the preparative reserve-phase chromatographic separation of oligoribonucleotides. Acta Chem. Scand. B 1984, 38, 657–671. [Google Scholar]
- Artal, M.C.; Toyne, K.J.; Goodby, J.W.; Barberá, J.; Photinos, D.J. Synthesis and mesogenic properties of novel board-like liquid crystals. J. Mater. Chem 2001, 11, 2801–2807. [Google Scholar]
- Kaller, M.; Tussetschläger, S.; Fischer, P.; Deck, C.; Baro, A.; Giesselmann, F.; Laschat, S. Columnar mesophases controlled by counterions in potassium complexes of dibenzo[18]crown-6 derivatives. Chem. Eur. J 2009, 15, 9530–9542. [Google Scholar]
- Yasuda, T.; Shimizu, T.; Liu, F.; Ungar, G.; Kato, T. Electro-functional octupolar π-conjugated columnar liquid crystals. J. Am. Chem. Soc 2011, 133, 13437–13444. [Google Scholar]
- Martinez-Palau, M.; Perea, E.; Lopez-Calahorra, F.; Velasco, D. Synthesis of luminescent N-arylcarbazoles by copper bronze-mediated reaction. Lett. Org. Chem 2004, 1, 231–237. [Google Scholar]
- Ma, C.-Q.; Pisula, W.; Weber, C.; Feng, X.-L.; Müllen, K.; Bäuerle, P. Dendritic oligothiophenes terminated with tris(alkyloxy)phenylethynyl tails: Synthesis, physical properties, and self-assembly. Chem. Eur. J 2011, 17, 1507–1518. [Google Scholar]
- Lee, H.; Kim, D.; Lee, H.-K.; Qiu, W.; Oh, N.-K.; Zin, W.-C.; Kim, K. Discotic liquid crystalline materials for potential nonlinear optical applications: synthesis and liquid crystalline behavior of 1,3,5-triphenyl-2,4,6-triazine derivatives containing achiral and chiral alkyl chains at the periphery. Tetrahedron Lett 2004, 45, 1019–1022. [Google Scholar]
- Jung, M.E.; Tsvetelina, I.; Lazarova, T.I. New Efficient Method for the Total Synthesis of (S,S)-Isodityrosine from Natural Amino Acids. J. Org. Chem 1999, 64, 2976–2977. [Google Scholar]
- Beckmann, J.; Dakternieks, D.; Andrew Duthie, A.; Allan, E.K.; Lim, A.E.K.; Tiekink, E.R.T. Ring strain in boroxine rings: computational and experimental considerations. J. Organomet. Chem 2001, 633, 149–156. [Google Scholar]
- Prasad, S.K.; Rao, D.S.S.; Chandrasekhar, S.; Kumar, S. X-Ray studies on the columnar structures of discotic liquid crystals. Mol. Cryst. Liq. Cryst 2003, 396, 121–139. [Google Scholar]
- Snyder, H.R.; Konecky, M.S.; Lennarz, W.J. Aryl Boronic Acids. II. Aryl Boronic Anhydrides and their Amine Complexes. J. Am. Chem. Soc 1958, 80, 3611–3615. [Google Scholar]







| Compound | Phase | Tm [°C] (ΔH [kJ·mol−1]) | Phase | Tc [°C] (ΔH [kJ·mol−1]) | Phase | Cycles |
|---|---|---|---|---|---|---|
| 11a | Cr | 24 | – | – | I | 2nd heating |
| 11b | Cr | 25 (65.5) | Colh | 135 c (3.4) | I | 2nd heating |
| 11b | Cr | 7 (25.2) | Colh | 129 c (1.3) | I | 2nd cooling |
| 11c | Cr | 30 (84.3) | Colh | 130 c (7.7) | I | 2nd heating |
| 11c | Cr | 22 (73.4) | Colh | 127 c (4.4) | I | 2nd cooling |
| 11d | Cr | 42 (116.4) | Colh | 128 c (2.3) | I | 2nd heating |
| 11d | Cr | 24 (72.7) | Colh | 126 (2.5) | I | 2nd cooling |
| 11e | Cr | 49 (123.9) | Colh | 126 c (5.6) | I | 2nd heating |
| 11e | Cr | 33 (86.1) | Colh | 123 (3.3) | I | 2nd cooling |
aThe following phases were observed: crystalline (Cr), columnar hexagonal (Colh), isotropic liquid (I)btransition temperatures were determined by DSC (heating/cooling rate 5 K·min−1)cpeak temperature.
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wöhrle, T.; Baro, A.; Laschat, S. Novel Discotic Boroxines: Synthesis and Mesomorphic Properties. Materials 2014, 7, 4045-4056. https://doi.org/10.3390/ma7054045
Wöhrle T, Baro A, Laschat S. Novel Discotic Boroxines: Synthesis and Mesomorphic Properties. Materials. 2014; 7(5):4045-4056. https://doi.org/10.3390/ma7054045
Chicago/Turabian StyleWöhrle, Tobias, Angelika Baro, and Sabine Laschat. 2014. "Novel Discotic Boroxines: Synthesis and Mesomorphic Properties" Materials 7, no. 5: 4045-4056. https://doi.org/10.3390/ma7054045