Summary
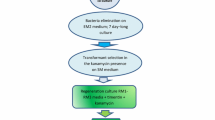
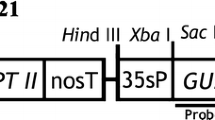
Genetic transformation systems have been established for Brassica nigra (cv. IC 257) by using an Agrobacterium binary vector as well as by direct DNA uptake of a plasmid vector. Both the type of vectors carried nptII gene and gus gene. For Agrobacterium mediated transformation, hypocotyl tissue explants were used, and up to 33% of the explants produced calli on selection medium. All of these expressed B-glucuronidase gene on histochemical staining. Protoplasts isolated from hypocotyl tissues of seedlings could be transformed with a plasmid vector by FEG mediated uptake of vector DNA. A number of fertile kanamycin resistant plants were obtained using both the methods, and their transformed nature was confirmed by Southern blot analysis and histochemical staining for GUS. Backcrossed and selfed progenies of these transformed plants showed the presence of npt and gus genes.
Similar content being viewed by others
References
Barfield DG, Pua E-C (1991) Gene transfer in plants of Brassica juncea using. Agrobacterium tumefaciens — mediated transformation. Plant Cell Rep 10: 308–314
Charest PJ, Holbrook LA, Gabard J, Iyer VN, Miki BL (1988) Agrobacterium-mediated transformation of thin cell layer explants from Brassica napus L. Theor Appl Genet 75: 438–445
De Block M, De Brouwer D, Tenning P (1989) Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of bar and neo genes in the transgenic plants. Plant Physiol 91: 694–701
Dellaporta SL, Wood J, Hicks JB (1984) A plant DNA minipreparation. In: A Laboratory Course Manual, Cold spring Harbor Laboratory, Cold Spring Harbor, New York, pp 36–37
Everett NP, Robinson KEP, Mascarenhas D (1987) Genetic engineering of sunflower (Helianthus annus L.). Bio/Technology 5: 1201–1204
Fry J, Barnason A, Horsch RB (1987) Transformation of Brassica napus with Agrobacterium tumefaciens based vectors. Plant Cell Rep 6: 321–325
Glimelius K (1984) High growth rate and regeneration capacity of hypocotyl protoplasts in some Brassicaceae. Physiol Plant 61: 38–44
Golz C, Kohler F, Sacristan MD, Schieder O (1988) Transformation of Brassica species via direct gene transfer. In: Puite J, Dons JJM, Huizing HJ, Kool AJ, Koorneef M, Krens FA (Eds) Progress in plant protoplast research. Kluwer, Dordrecht, pp 353–354
Golz C, Kohler F, Schieder O (1990) Transfer of hygromycin resistance into Brassica napus using total DNA of a transgenic B. nigra line. Plant Mol Biol 15: 475–483
Guerche P, Charbonnier M, Jounain L, Tourneur C, Paszkowski J, Pelletier G (1987) Direct gene transfer by electroporation in Brassica napus. Plant Sci 52: 111–116
Guerche P, De Almedia ERP, Schwarztein MA, Gander E, Krebbers E, Pelletier G (1990) Expression of the 2S albumin from Bertholletia excelsa in Brassica napus. Mol Gen Genet 221: 306–314
Gupta V, Agnihotri A, Jagannathan V (1990a) Plant regeneration from callus and protoplasts of Brassica nigra (IC 257) through somatic embryogenesis. Plant Cell Rep 9: 427–430
Gupta V, Jagannathan V, Lakshmikumaran MS (1990b) A novel AT-rich tandem repeat of Brassica nigra. Plant Sci 68: 223–229
Horn ME, Shillito RD, Conger BV, Harms CT (1988) Transgenic plants of orchardgrass (Dactylis glomerata) from protoplasts. Plant Cell Rep 7: 469–472
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 386–396
Lorz H, Baker B, Schell J (1985) Gene transfer to cereal cells mediated by protoplast transformation. Mol Gen Genet 199: 178–182
Mathews H, Bharathan N, Litz RE, Narayanan KR, Rao PS, Bhatia CR (1990) Transgenic plants of mustard Brassica juncea (L.) Czern and Coss. Plant Sci 72: 245–252
Menczel L, Nagy I, Kizz ZR, Maliga P (1981) Streptomycin resistant and sensitive somatic hybrids of Nicotiana tabacum + Nicotiana knightiana: correlation of resistance to N. tabacum plastids. Theor Appl Genet 59: 191–195
Moloney MM, Walker JM, Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8: 238–242
Mukhopadhyay A, Arumugam N, Nandakumar PBA, Pradhan AK, Gupta V, Pental D (1992) Agrobacterium mediated genetic transformation of oilseed Brassica campestris: Transformation frequency is strongly influenced by the mode of shoot regeneration. PlantCell Rep 10: 506–513
Mukhopadhyay A, Topfer R, Pradhan AK, Sodhi YS, Steinbis HH, Schell J, Pental D (1991) Efficient regeneration of Brassica leracea hypocotyl protoplasts and high frequency genetic ransformation by direct DNA uptake. Plant Cell Rep 10: 375–379
Pua E-C, Mehra-Palta A, Nagy F, Chua N-H (1987) Transgenic plants f Brassica napus L. Bio/Technology 5: 815–817
Radke SE, Andrews BM, Moloney MM, Crouch ML, Kridl JC, Knauf VC (1988) Transformation of Brassica napus L. using Agrobacterium tumefaciens: developmentally regulated expression of a reintroduced napin gene. Theor Appl Genet 75: 685–694
Sacristan MD, Gerdemann-Knorck M, Schieder O (1988) Transformation of Brassica nigra through protoplast cocultivation with Agrobacterium tumefaciens In: Puite KJ, Dons JJM, Huizing HJ, Kool AJ, Koorneef M, Krens FA (Eds), Progress in plant protoplast research. Kluwer, Dordrecht pp 252–253
Sacristan MD, Gerdemann-Knorck M, Schieder O (1989) Incorporation of hygromycin resistance in Brassica nigra and its transfer to B. napus through asymmetric protoplast fusion. Theor Appl Genet 78: 194–200
Saul MW, Shillito RD, Negrutiu I (1988) Direct DNA transfer to protoplasts with and without electroporation. Plant Mol Biol Manual A1: 1–16
Srivastava V, Reddy AS, Guha-Mukherjee S (1988) Transformation and regeneration of Brassica oleracea mediated by an oncogenic Agrobacterium tumefaciens. Plant Cell Rep 7: 504–507
Thomzik JE, Hain R (1990) Transgenic Brassica napus plants obtained by cocultivation of protoplasts with Agrobacterium tumefaciens. Plant Cell Rep 9: 233–236
Topfer R, Gronenborn B, Schell J, Steinbiss H H (1989) Uptake and transient expression of chimeric genes in seed-derived embryos. The Plant Cell 1: 133–139
Topfer R, Schell J, Steinbis HH (1988) Versatile cloning vectors for transient gene expression and direct gene transfer in plants. Nucl Acids Res 16: 8725
Toriyama K, Arimoto Y, Uchimiya H, Hinata K (1988) Transgenic rice plants after direct gene transfer into protoplasts. Bio/Technology 6: 1072–1074
UN (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japan J Bot 7: 389–452
Zhang HM, Yang H, Rech EL, Golds TJ, Davis AS, Mulligan BJ, Cocking EC, Davey MR (1988) Transgenic rice plants produced by electroporation-mediated plasmid uptake into protoplasts. Plant Cell Rep 7: 379–384
Author information
Authors and Affiliations
Additional information
Communicated by E. D. Earle
Rights and permissions
About this article
Cite this article
Gupta, V., Lakshmi Sita, G., Shaila, M.S. et al. Genetic transformation of Brassica nigra by agrobacterium based vector and direct plasmid uptake. Plant Cell Reports 12, 418–421 (1993). https://doi.org/10.1007/BF00234704
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00234704